When you pick up a prescription, you might see two options: the name you recognize from TV ads, and a much cheaper version with a strange chemical name. That’s the generic vs brand drug choice-and it’s one of the biggest ways you can save money on healthcare without sacrificing effectiveness.
What exactly is a generic drug?
A generic drug is the exact same medicine as the brand-name version, just sold under a different name after the patent expires. It contains the same active ingredient, in the same strength, same dosage form, and works the same way in your body. The FDA requires it to be bioequivalent-meaning it delivers the same amount of medicine into your bloodstream at the same rate as the brand. That’s not a guess. It’s a legal requirement backed by lab tests and clinical data.
Think of it like buying store-brand cereal instead of the name-brand one. Same ingredients, same nutrition, different packaging and price. The FDA inspects generic drug factories just as often as brand-name ones. In fact, many brand-name companies actually make their own generics under different labels. So the fear that generics are "second-rate" isn’t just wrong-it’s backwards.
How much cheaper are generics really?
On average, generic drugs cost 79% to 85% less than their brand-name equivalents. That’s not a small difference. A 30-day supply of a brand-name blood pressure pill might cost $120. The generic? Around $20. For insulin, the gap can be thousands of dollars a year.
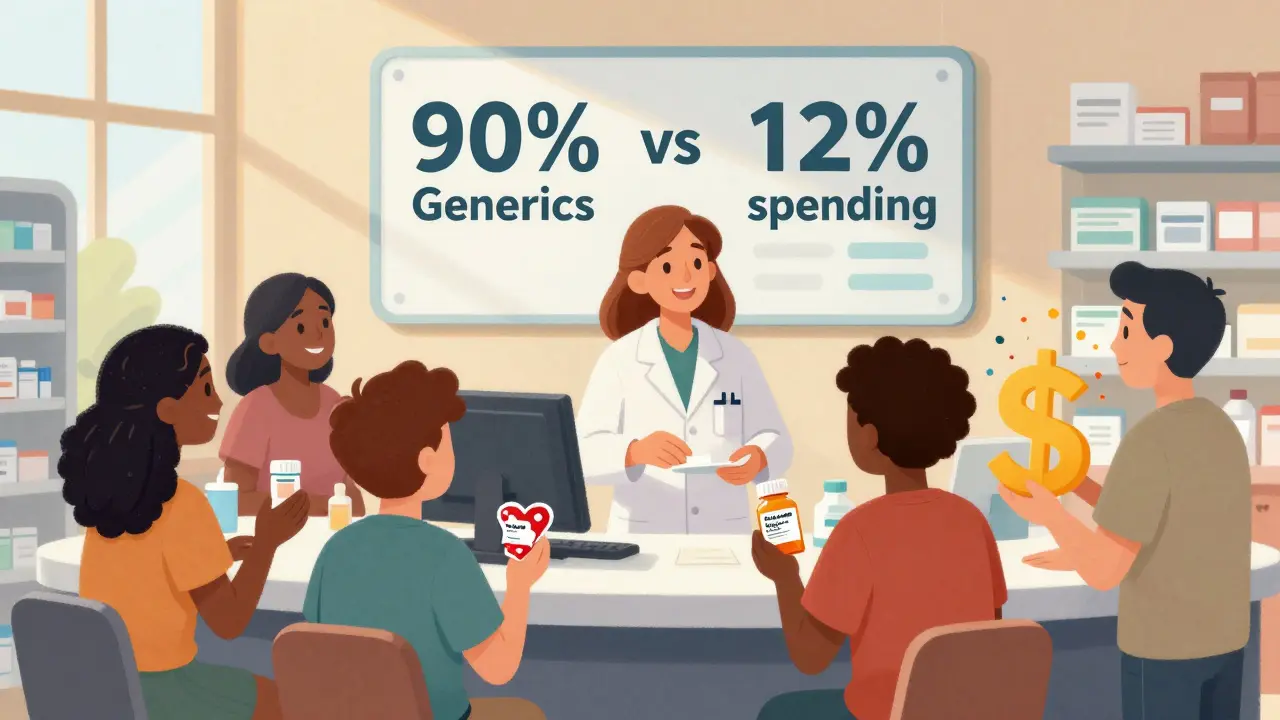
In 2024, generics made up 90% of all prescriptions filled in the U.S.-but only 12% of total drug spending. That means Americans filled 3.9 billion generic prescriptions for $98 billion, while just 435 million brand-name prescriptions cost $700 billion. The math is clear: generics are the reason prescription costs haven’t exploded even more.
And it gets even better. The more generic versions of a drug hit the market, the cheaper it gets. With just one generic competitor, prices drop to about 90% of the original brand price. With three or four, they fall to 60-70%. With five or more, they often drop below 50% of the original price. That’s why drugs like Nexavar and Januvia saw massive price cuts from their makers once generics arrived-even before the law forced them to.
Why do brand-name drugs cost so much?
Brand-name companies spend a lot on research, clinical trials, and marketing. That’s why they get a patent-to recoup those costs and make a profit. But here’s the twist: once the patent expires, those costs are already paid. The generic makers don’t need to repeat expensive trials. They just need to prove their version works the same. That’s why they can sell it for pennies.
But here’s what most people don’t realize: brand-name drug prices have been falling too. In 2025, Bayer cut Nexavar’s list price by 50% after the first generic came out. Merck dropped Januvia’s price by 42.4%. Why? Because they knew they couldn’t keep charging $1,000 a month when a $20 generic was available. They’re lowering prices to stay competitive, or to keep their rebate deals with insurers.
And even though list prices might look high, what you actually pay-called the "net price"-is often much lower because of rebates and discounts. In 2024, the gap between list and net prices was the smallest it’s been in years. That means manufacturers are giving bigger discounts just to keep their drugs on insurance formularies.
Are generics really as good as brand-name drugs?
Yes. And the evidence is overwhelming.
The FDA requires generics to meet the same strict standards as brand-name drugs for quality, purity, strength, and stability. They’re tested in the same labs. They’re held to the same manufacturing rules. The American Medical Association, the FDA, and top medical schools all agree: generics are therapeutically equivalent.
But perception is still a barrier. A 2025 survey found that 84% of Americans believe generics are just as effective. Yet 62% still trust brand-name drugs more. Why? Because they’ve been told for decades that "name matters." Marketing works. TV ads make you feel safer with the brand. But the science doesn’t care about the label.
Real-world studies back this up. One analysis of over 100,000 patients taking generic versions of heart medications found no difference in hospitalizations or deaths compared to those on brand names. Another study on antidepressants showed identical outcomes. If your doctor says the generic is safe, it is.
Why do some people still choose brand-name drugs?
Cost is the biggest reason people pick generics-63% say they choose them because they can’t afford the brand. But 60% of those same people admit they’d prefer the brand if money weren’t an issue. That’s not about effectiveness. It’s about anxiety.
Some people worry about inactive ingredients-fillers, dyes, coatings. These can vary between brands and generics, and in rare cases, they might cause a reaction in someone with a severe allergy. But that’s not about the medicine working. It’s about a reaction to something that doesn’t do anything medically. If you’ve had a reaction before, tell your pharmacist. They can check the ingredients.
Another reason? Habit. If you’ve been taking a brand-name drug for years and your doctor didn’t mention switching, you might just keep taking it. But that doesn’t mean it’s better. It just means you haven’t been asked to consider the cheaper option.

What’s changing in 2025 and beyond?
The Inflation Reduction Act is changing the game. Starting in January 2026, Medicare will cap the price of 10 high-cost drugs at $35 per month. Many of those drugs are brand-name, and many will have generics available. That means even more pressure on manufacturers to lower prices.
Also, Medicare Part D is being redesigned. By 2025, your out-of-pocket drug costs will be capped at $2,000 a year. That’s huge. It means people won’t have to choose between buying medicine and paying rent. And with generics being so cheap, they’ll be the go-to option for most.
Meanwhile, pharmaceutical companies are shifting strategies. Instead of fighting generics, some are lowering their own prices slowly to stay relevant. Others are bundling their brand drugs with support services-like free monitoring or nurse hotlines-to justify the higher cost. But for most people, the math still favors the generic.
How to switch to a generic drug
Switching is easier than you think.
- Ask your doctor: "Is there a generic version of this drug?" They might already have one in mind.
- Check with your pharmacist. They can tell you if a generic exists and how much it costs.
- Call your insurance. Some plans require you to try the generic first.
- Don’t be afraid to ask for a switch. Doctors expect this question.
- If you’ve had issues before-like side effects or allergic reactions-tell your provider. They’ll help you decide.
Most switches happen without any problems. If you do notice a change-like a different side effect or feeling off-talk to your doctor. But it’s rarely because the generic didn’t work. It’s usually your body adjusting to a new pill shape, color, or inactive ingredient.
Bottom line: Choose generic unless there’s a real reason not to
There’s no medical reason to pay more for the brand name. Generics are just as safe. Just as effective. And 80% cheaper. The system is built so that brand-name drugs fund innovation, and generics make sure that innovation is accessible to everyone.
Every time you choose a generic, you’re not just saving money. You’re helping keep healthcare affordable for everyone else too. The U.S. spends less per prescription than countries like Canada and Germany-not because we have cheap brand drugs, but because we have the cheapest generics in the world.
Next time you get a prescription, ask: "Is there a generic?" The answer might save you hundreds-or even thousands-this year.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same strict standards for safety, strength, quality, and purity as brand-name drugs. They’re tested in the same way and inspected in the same facilities. The active ingredient is identical. The only differences are in inactive ingredients like dyes or fillers-which rarely cause issues and are listed on the label.
Why do generics cost so much less?
Generic manufacturers don’t have to repeat expensive clinical trials. Once a brand-name drug’s patent expires, other companies can apply to make a version that’s bioequivalent-proving it works the same way without starting from scratch. That cuts development costs dramatically. They also don’t spend millions on TV ads or celebrity endorsements.
Can I trust generics from other countries?
Only if they’re sold in the U.S. and approved by the FDA. Drugs imported from other countries aren’t regulated by the FDA and may not meet U.S. safety standards. Stick to generics sold by U.S. pharmacies-whether they’re made in the U.S., India, or elsewhere-as long as they’re FDA-approved.
Do generics take longer to work?
No. The FDA requires generics to be bioequivalent, meaning they deliver the same amount of active ingredient into your bloodstream at the same rate as the brand. Any difference in how fast you feel the effect is usually psychological or due to changes in inactive ingredients, not the medicine itself.
What if my insurance won’t cover the generic?
That’s rare, but it can happen. Some insurance plans require prior authorization for generics, or may have a tiered system where the brand is cheaper. If this happens, ask your pharmacist to check if there’s a different generic option or if your doctor can submit an appeal. You can also ask for a 30-day trial of the generic to see if it works for you before committing.
Are all brand-name drugs available as generics?
Most are, but not all. Newer drugs are still under patent protection-usually for 10 to 12 years after approval. Some complex drugs, like biologics, have a longer path to generic versions called biosimilars, which are similar but not identical. For most common medications-like blood pressure pills, statins, or antibiotics-a generic is already available.


Alex LaVey
February 3, 2026
Just switched my dad to generic lisinopril last month-he was paying $110 a month for the brand. Now it’s $18. He didn’t notice any difference, and his BP is actually more stable. I wish more people knew this stuff. Healthcare doesn’t have to be a scam if you know where to look.
rahulkumar maurya
February 5, 2026
Let us not mince words: the American pharmaceutical industry is a grotesque parody of capitalism. Brand-name drugs are not 'innovations'-they are rent-seeking monopolies propped up by regulatory capture. Generics? The only honest product in the entire supply chain. The fact that 90% of prescriptions are generic yet they account for 12% of spending? That’s not a coincidence. That’s justice.
Meanwhile, the average American still believes 'name brand = better' because they’ve been conditioned by billion-dollar ad campaigns targeting their insecurities. Pathetic. And yet, here we are.
Also, if you're worried about 'inactive ingredients,' please tell me you're not the same person who thinks organic kale is magically healthier than conventional kale. Science doesn't care about your marketing-induced anxiety.
Demetria Morris
February 6, 2026
I used to refuse generics because I thought they were 'cheap'-like buying store-brand toilet paper. Then my insurance forced me to switch to generic metformin. I was terrified. But nothing changed. Not my energy, not my digestion, not my blood sugar. I felt stupid for ever doubting it. Now I tell everyone: if your doctor says it’s safe, it is. Stop paying for the logo.
Susheel Sharma
February 7, 2026
Generics are the only reason the U.S. hasn’t collapsed under its own healthcare debt. 😅
Let’s be real-brand names are just fancy packaging for the same chemical you can buy for 1/5th the price. The fact that people still pay full price? That’s not ignorance. That’s willful compliance with a broken system. Pharma companies don’t want you to know this. They want you to fear the unknown. But the unknown is just... the same pill. In a different box.
Also, 'biosimilars' are not generics. Don’t confuse the two. That’s a whole other scam.
Roshan Gudhe
February 8, 2026
There’s a quiet revolution happening in medicine-and most people don’t even know it’s happening. We’ve built a system where innovation is funded by the suffering of the poor. Generics are the counterweight. They’re not just cheaper-they’re ethical. Choosing a generic isn’t about saving money. It’s about refusing to participate in a system that profits from fear.
Think about it: if a drug can be made for $20 and sold for $120, who’s really paying the cost? Not the manufacturer. Not the pharmacist. You. And your neighbor. And your parent on Medicare.
Maybe the real question isn’t 'Are generics safe?' but 'Why do we still allow this pricing model to exist?'
Rachel Kipps
February 8, 2026
i switched to generic omeprazole last year and my stomach felt weird for like a week? i thought it was the pill but then i realized i had just started drinking coffee again. my bad. generics are fine. just ask your doc or pharmacist if you’re unsure. they’re not trying to trick you.
Prajwal Manjunath Shanthappa
February 9, 2026
Oh, so now we’re supposed to believe that a pill with a different color and shape is 'just as good'? How naive. The FDA doesn’t test for psychological effects. What if the generic makes you feel less confident? What if the placebo effect is the only thing keeping your anxiety under control? You think science is everything? You’re ignoring the human element.
And don’t get me started on Indian generics-how do you know they’re not using substandard manufacturing? The FDA can’t be everywhere! This is reckless!
...I’m just saying, if you’re going to gamble with your health, at least pay for the branded version. You owe it to yourself.
Wendy Lamb
February 10, 2026
My pharmacist just told me my brand-name thyroid med has a generic now. I didn’t even know. I’ve been paying $80 for years. It’s $12. I’m switching tomorrow. Thank you for this post.