When a drug company spends billions to develop a new medicine, it makes sense they’d want to protect their investment. But what happens when that protection stretches for decades-not because of real innovation, but because of clever legal tweaks? That’s evergreening-a strategy used by big pharmaceutical companies to keep generics off the market long after a drug’s original patent should have expired.
What Exactly Is Evergreening?
Evergreening isn’t about inventing new drugs. It’s about making tiny changes to old ones-like switching from a pill to a liquid, changing the dosage, or combining it with another ingredient-and then filing a new patent. These aren’t breakthroughs. They’re tweaks. But under U.S. patent law, even minor modifications can trigger a fresh 20-year clock on exclusivity.
The system was designed to balance innovation and access. The 1984 Hatch-Waxman Act let generic manufacturers enter the market after patents expired, lowering prices dramatically. But drug companies quickly learned how to game it. Instead of waiting for generics to arrive, they started layering patents-like stacking bricks-to build a wall around their best-selling drugs.
How It Works: The Playbook
Here’s how it typically plays out:
- A drug gets approved. Its main patent lasts 20 years from filing.
- Five years before expiration, the company files for a new patent on a slightly different version-say, a slow-release pill instead of a regular one.
- The FDA grants three extra years of exclusivity because the company ran new clinical trials (even if they just tested absorption rates).
- Then they file another patent for a pediatric use, adding six more months.
- They launch a branded version of the generic they themselves own-called an “authorized generic”-to keep profits flowing while pretending to allow competition.
- By the time the original patent expires, the company has built a web of 10, 20, even 50+ overlapping patents.
This isn’t theory. It’s standard practice. AstraZeneca filed patent protections for six top drugs, extending their monopoly by over 90 years combined. Humira, AbbVie’s blockbuster drug for autoimmune diseases, has more than 247 patents attached to it. Over 100 have already been granted. That’s not innovation. That’s legal engineering.
The Real Cost: Patients Pay the Price
When generics enter the market, prices drop by 80-85% within a year. That’s the whole point of the system. But evergreening delays that drop-sometimes for a decade or more.
Take Humira. It was approved in 2002. The original patent expired in 2016. But because of patent stacking, generics didn’t hit the U.S. market until 2023. In the meantime, patients paid $7,000-$9,000 per month. AbbVie made over $40 million a day from it. That’s not luck. That’s strategy.
And it’s not just Humira. AstraZeneca’s Nexium, a reformulation of Prilosec, became the #1 best-selling drug in the world-not because it was better, but because the company convinced doctors and patients to switch. The new version had a slightly different chemical structure. It worked the same. But the patent clock reset. Patients didn’t save money. They paid more.
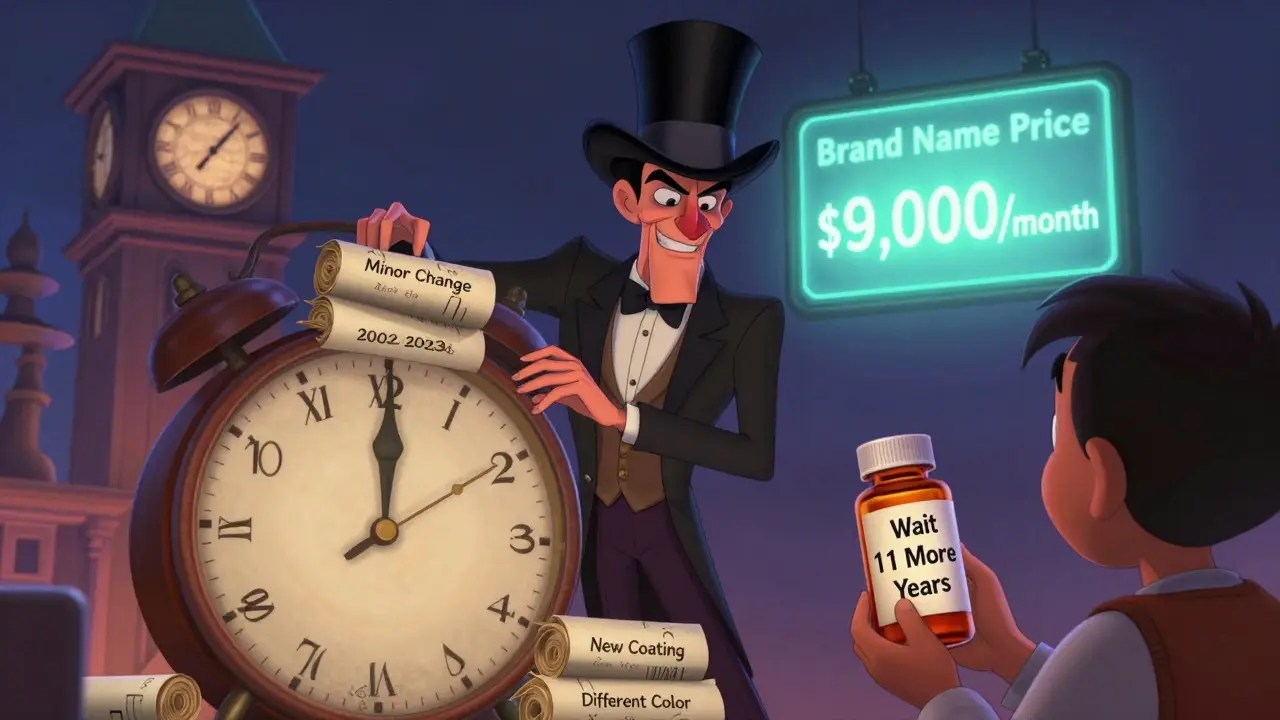
Why Do Companies Do This?
Because it works. And it’s cheap.
Developing a brand-new drug costs about $2.6 billion and takes 10-15 years. Evergreening? A fraction of that. A new formulation might cost $50 million to test. The return? Billions in extra revenue. And since the FDA and patent office often approve these minor changes without demanding real clinical improvement, companies have little incentive to stop.
Robin Feldman, a law professor who’s studied this for years, found that 78% of new patents on prescription drugs are for existing medications-not new ones. That’s the industry’s secret: innovation isn’t the business model. Profit protection is.
Regulators Are Starting to Push Back
But the tide is turning.
In 2022, the U.S. Federal Trade Commission sued AbbVie over Humira’s patent strategy, calling it an illegal monopoly. The Inflation Reduction Act of 2022 gave Medicare the power to negotiate prices for the most expensive drugs, cutting into the profits of evergreened products. The European Medicines Agency now requires proof of real clinical benefit before granting extra exclusivity.
Even the U.S. Patent Office is getting stricter. Examiners are now rejecting “obvious” patents-like changing a pill’s color or coating-more often than before. Courts are also starting to strike down patent thickets when they’re clearly designed to block competition.
Still, the system isn’t fixed. Generic manufacturers still face years of lawsuits, delays, and legal costs just to get their products approved. Many can’t afford the fight.
What’s Next? Biologics and Beyond
The next frontier for evergreening? Biologics-complex drugs made from living cells, like insulin or cancer treatments. These are harder to copy than pills, so companies are using that to their advantage.
Some are patenting genetic tests that predict who will respond to a drug. Others are creating nanotech versions that are nearly impossible for generics to replicate. A few are even using data exclusivity rules to block generic makers from using clinical trial data-even if they do their own studies.
These aren’t science fiction. They’re happening now. And without stronger oversight, they’ll become the new normal.
Who Gets Hurt?
Patients. Especially those without good insurance. People with diabetes, arthritis, Crohn’s disease, and other chronic conditions can’t choose between rent and their medication. Evergreening turns life-saving drugs into luxury items.
It also strains public health systems. Medicaid, Medicare, and VA hospitals pay billions more each year because generics are delayed. Taxpayers foot the bill. And in low-income countries, where these drugs are priced out of reach, the impact is even worse.
There’s no moral justification here. No public health benefit. Just profit.
Is There a Better Way?
Yes. And it’s simple: reward real innovation, not legal loopholes.
Instead of extending patents for minor changes, governments could offer cash prizes for breakthrough drugs. Or create a public fund that pays companies for developing truly new treatments-then let everyone make generics right away.
Some countries already do this. The U.K. and Canada have experimented with prize systems. They don’t rely on monopolies. They reward discovery, not delay.
Until the U.S. and other major markets adopt similar models, evergreening will keep thriving. And patients will keep paying the price.
Is evergreening legal?
Yes, it’s currently legal in the U.S. and many other countries. The patent system allows companies to file for new patents on modified versions of existing drugs-even if the changes are minor. While courts and regulators are starting to challenge some of these tactics, there’s no law that outright bans evergreening. The loophole exists because the system was designed to encourage innovation, not to prevent strategic patenting.
How does evergreening affect generic drug prices?
It delays price drops. Once a generic enters the market, prices typically fall by 80-85% within a year. But evergreening can push that moment back by 5, 10, or even 15 years. During that time, patients pay full brand-name prices. In some cases, companies even release their own "authorized generics"-sold under the brand name-to keep profits high while pretending to allow competition.
What’s the difference between evergreening and real innovation?
Real innovation creates a new medicine with a new mechanism of action, targeting a disease differently or offering better outcomes. Evergreening changes the delivery method, dosage, or adds a second ingredient-without proving better results. For example, switching from a pill to a patch or changing the release time doesn’t make a drug more effective. But it can reset the patent clock. The difference is: one saves lives. The other saves profits.
Which drugs are most affected by evergreening?
The biggest targets are blockbuster drugs with high sales volume and low production cost. These include Humira (for autoimmune diseases), Nexium (for acid reflux), Lipitor (for cholesterol), and drugs for diabetes like Januvia. AstraZeneca, AbbVie, and Pfizer have been the most aggressive in using this strategy. These drugs often treat chronic conditions, meaning patients take them for years-making them perfect targets for long-term profit.
Can the government stop evergreening?
Yes-but it’s hard. The U.S. Patent and Trademark Office can reject obvious patents. The FDA can demand proof of real clinical benefit. Courts can strike down patent thickets. The FTC can sue for antitrust violations. And laws like the Inflation Reduction Act are starting to reduce the financial incentive by capping prices. But each of these tools moves slowly, and drug companies have armies of lawyers and lobbyists. Real change requires systemic reform, not just case-by-case challenges.


THANGAVEL PARASAKTHI
February 8, 2026
Man, i read this and just felt so damn frustrated. In india, we see so many people skipping meds because they cost too much. And here? Companies just sit on patents like they own the damn air. It’s not innovation. It’s greed wrapped in a lab coat.
Frank Baumann
February 8, 2026
Let me tell you something-this isn’t just a pharmaceutical problem, this is a systemic rot in the entire American legal and economic framework. We’ve turned healthcare into a casino where the house always wins, and the players? They’re the ones with diabetes, asthma, rheumatoid arthritis-people who can’t afford to lose. And the FDA? They’re not regulators-they’re gatekeepers for corporate lobbyists. You think the patent office is impartial? Ha. They’re paid in campaign donations and fancy dinners. This isn’t capitalism. This is feudalism with a pharmacy logo.
Scott Conner
February 8, 2026
wait so if a company just changes the color of a pill or makes it dissolve slower, they get another 20 years? that’s insane. i thought patents were for actual inventions. not… marketing tricks. is there any law that says you have to prove it’s *better*, not just *different*? because if not, that’s the flaw right there.
Susan Kwan
February 9, 2026
Oh wow, so the drug companies didn’t invent a cure-they invented a *legal loophole*? Shocking. Next they’ll patent oxygen and charge us for breathing. At this point, I’m just waiting for the day they sue me for using my own immune system.
Random Guy
February 10, 2026
Humira. $7,000 a month. For 7 years. That’s like paying for a luxury SUV… every 3 weeks. And the company? They didn’t even *improve* the drug. They just changed the damn packaging. I swear, if you gave these CEOs a rubber duck and told them to patent it, they’d file 12 patents on ‘duck-shaped floatation device with enhanced ergonomic grip.’
Tasha Lake
February 11, 2026
From a regulatory science standpoint, the Hatch-Waxman Act created a brilliant equilibrium between innovation incentives and market access-but the industry exploited the 3-year exclusivity window for ‘new formulations’ and the 6-month pediatric extension as structural arbitrage opportunities. What we’re seeing now is patent thickening-a strategic aggregation of IP rights to create non-obvious barriers to market entry, effectively functioning as regulatory capture. The FDA’s lack of clinical superiority requirements for reformulations is the critical failure point.
Brett Pouser
February 11, 2026
Man, I’ve got a cousin with Crohn’s. She’s been on Humira since she was 21. Now she’s 30. She’s had to switch jobs three times just to keep insurance. I never realized how much of her life was dictated by a patent expiration date. This isn’t business. This is cruelty dressed up as ‘market strategy.’
Karianne Jackson
February 12, 2026
So they just make tiny changes and call it new? That’s like saying a red crayon is a totally different color than a pink one. We’re being scammed. Plain and simple.
Andy Cortez
February 12, 2026
Oh here we go again. The ‘evil pharma’ narrative. Wake up. Without patents, no one would invest in ANY drug. Ever. You want generics? Fine. But then you get zero new treatments. Cancer cures? Gone. Alzheimer’s meds? Never invented. This isn’t greed-it’s the only thing keeping innovation alive. You can’t have your cake and eat it too.
Jacob den Hollander
February 14, 2026
I hear you, Andy-but the problem isn’t patents. It’s *abuse* of patents. If you invent a new drug that saves lives? Totally deserve protection. But if you just change the pill’s shape and call it ‘advanced delivery’? That’s not innovation. That’s gaming the system. Real innovation deserves reward. Manipulation deserves scrutiny. There’s a difference.
John Sonnenberg
February 14, 2026
Let’s not pretend this is about patients. This is about quarterly earnings reports. Executives get bonuses based on revenue growth, not health outcomes. If a CEO can make $2 billion more by delaying a generic, they will. Every. Single. Time. And shareholders cheer. That’s not capitalism-it’s a Ponzi scheme with a prescription pad.
PAUL MCQUEEN
February 16, 2026
So what? The market works. If people can’t afford it, they shouldn’t take it. Maybe they should get a better job. Or move to a country with cheaper drugs. Not everyone gets a free ride. This isn’t a crisis. It’s economics.
glenn mendoza
February 16, 2026
While I acknowledge the systemic challenges presented by the current patent framework, I would respectfully submit that a more constructive path forward lies in public-private partnership models that incentivize true therapeutic innovation while simultaneously ensuring equitable access. A prize-based system, as implemented in certain OECD jurisdictions, may offer a more ethically coherent and economically sustainable alternative to the existing rent-seeking paradigm.
Kathryn Lenn
February 18, 2026
Of course it’s legal. The whole system is rigged. Big Pharma owns Congress, the FDA, the courts, the media. This isn’t a loophole-it’s the design. They’ve been doing this since the 70s. The ‘inflation reduction act’? A PR stunt. They’ll just move the patents offshore. Mark my words: next year, you’ll hear about ‘biologic data exclusivity in the Caymans.’ They’re always three steps ahead.
John Watts
February 18, 2026
You know what? This is why we need people to speak up. You read this? Share it. Talk to your reps. Tell your doctor. This isn’t just about drugs-it’s about dignity. People shouldn’t have to choose between insulin and rent. We can fix this. Not tomorrow. Not next year. But if enough of us push? Yeah. We can.